TWP’s Guide to the FDA-approved Drug Label
What is the Drug Label?
The FDA-approved “drug label” is a document written by the manufacturer of a particular pharmaceutical drug in collaboration with the U.S. Food and Drug Administration (FDA), and is intended to provide patients and practitioners with key information about one or more approved uses of the drug in the United States, its main chemical properties, some of its known risks, its interactions with other drugs and substances, and more. The official, complete FDA-approved drug label includes information about a drug that can be invaluable for understanding how the drug may be affecting you and for conducting the safest possible taper.
Drug labels in this and other countries may have other names such as the “drug product monograph”, and also sometimes go by the names of some of its component parts such as “Patient Package Insert”, “Medication Guide”, or “Full Prescribing Information”. Note that the documents ordinarily provided to consumers by pharmacists or found inside drug packages often include only a few pages of “highlights” from the full drug label or even unregulated, unapproved information. The full drug label, on the other hand, is mainly aimed at health professionals, and for psychiatric drugs can be 10-50 pages in length and sometimes longer. The drug label includes a great deal of important information; below, we explain how to get copies of drug labels, briefly summarize what is typically included in a drug label, and highlight those sections of a drug label that are of particular relevance when developing the safest possible tapering plan.
Sometimes, your physician has prescribed a drug to you for an “off-label” or “non FDA-approved” indication. That means, for example, that the manufacturer of the drug did not try or did not succeed to obtain FDA approval to market the drugs to people in your age group or who have the condition you’ve been diagnosed with. Thus the official drug label may not have information about possible drug effects on people such as yourself. Even in these situations, the complete FDA label of the drug you are taking will contain much vital information for understanding how the drug may be affecting you and for conducting the safest possible taper.
Where to Find Drug Labels
Some pharmacies will print out copies of a drug label upon request – be sure to get a copy of the complete drug label with the Full Prescribing Information and Medication Guide (where applicable) included, and not just 1-2 page summaries of the label in simplified language. These complete FDA-approved drug labels can also be viewed for free online. Some commercial sites provide access to altered versions of drug labels, but the most official, reliable and up-to-date labels are on the sites produced by the U.S. Federal government.
- U.S. National Library of Medicine “DailyMed” website
This website provides access to past and present drug labels for both brand-name and generic versions of drugs. It also sometimes provides links to related information, such as links to U.S. registered clinical trials.
- U.S. Food and Drug Administration “Drugs@FDA” database
This website provides access to “drug approval packages” for many drugs, which, along with drug labels, can also include historical documentation of medical reviews of clinical trials, statistical reviews, correspondence between the FDA and drug companies, and more. Note that only labels for brand-name drugs can be found here; no drug labels for generic drugs are available.
Search tips:
- Ask the pharmacist, or use a drug bottle, drug packet, and/or a copy of the drug product label that came with the drug to identify the drug’s official name. Note whether the name includes both a brand name and a generic name or just the generic name, and whether there are any supplements to the name such as acronyms or formulation descriptions (e.g. “Adderall XR” or “Depakote – Divalproex Sodium Delayed-release Tablets”). Use this complete name to conduct your search for the appropriate drug label.
- If a brand-name drug, search on the complete brand name (e.g. “Abilify (aripiprazole) tablet”).
- If a generic drug, when searching for the right drug label, match the name of the active ingredient or generic name for the drug and the packager and/or manufacturer’s name as well. However, if the drug label does not include all of the numbered sections described below, then it is likely an older label that has not been updated. It is advisable to then search to see if there is also a copy of a newer label for the same drug form and formulation from a different manufacturer.
- Always be sure that you are viewing the most recent version of the drug label. The date is usually found at the beginning or the end of the label, and sometimes in the margins. Also, different parts of the drug label may have different revision dates.
-
If you find the above websites and drug labels overwhelming or difficult to navigate, another option is to search for your drug in the database della U.S. Food and Drug Administration "Medication Guides". These Medication Guides are shorter, more streamlined versions of the drug labels, specifically designed to be more understandable to consumers. (As of 2018, many drugs still do not have a Medication Guide. And notably, where Medication Guides do exist, they are usually already included in a supplemental section of the full drug label.)
What’s Included in the Drug Label?
Newer drug labels often begin with one to two pages of highlights followed by a table of contents for the more detailed information that follows. Despite the large amount of information they contain, drug labels are not considered to be comprehensive, but are simply selective summaries of some of the most important issues that have come to the FDA’s attention about that particular drug and that have been decided to be worthy enough to be mentioned. In addition, the standards and requirements for the drug labels have changed over the years, so the subheaders, order of information, and amount of detail can vary greatly depending on how long the drug has been in the U.S. market and when the label was last updated. For these reasons, if a particular issue is not mentioned in a drug label, that cannot be taken to mean that the issue isn’t of concern in relation to that particular drug. Where your safety is at stake, please do further research and consult well-informed pharmacists and prescribers. Below, we identify the main sections of newer drug labels, along with a selective list of some of the types of useful information that can often be found in those sections – with a particular emphasis on information that can be important for tapering.
Typical Drug Label Contents
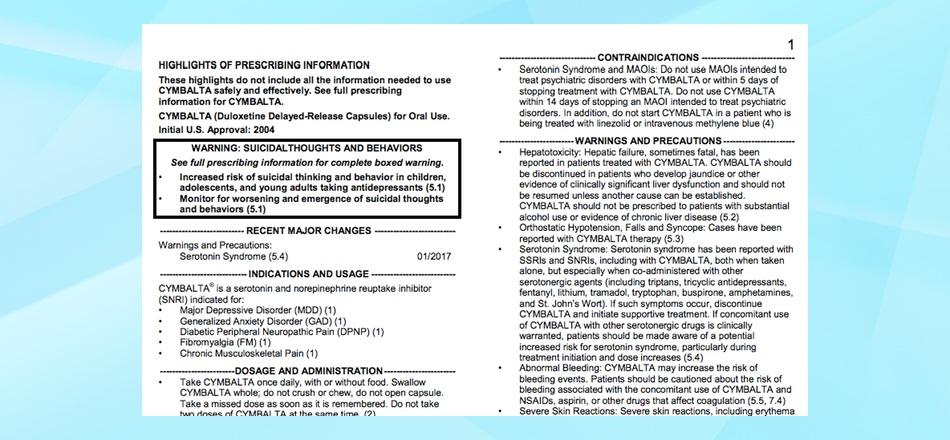
"Black Box" or "Boxed" Warnings

- If there is a particularly strong warning related to a serious risk linked to this drug, it is highlighted inside a black box or a bold-faced paragraph placed at the beginning of the label or after the table of contents.
1. Indications and Usage
- An overview of the “conditions” that the drug is approved by the FDA to treat.
- May also include very brief summaries of the drug trials that were submitted by the drug’s manufacturer to get it approved by the FDA for particular uses.
2. Dosage and Administration
- Information on recommended dosage amounts depending on various factors such as age, existing medical conditions, psychiatric labels, and other drugs taken.
- May also include information about dose adjustments to make depending on different genetic make-ups, how the drug is metabolized, and what other drugs a person may be taking or discontinuing.
- May include information about digestibility of beads outside of capsules, and/or how beads from opened capsules should and shouldn’t be consumed.
- May include information about equivalent dosages between modified-release and immediate-release formulations.
- May include information about the risks of discontinuing the drug.
- May contain information about how to discontinue the drug (Reminder: The discontinuation rates used in all FDA drug labels are considered dangerously fast and highly risky by most in the layperson withdrawal community.)
3. Dosage Forms and Strengths

- A listing of the different dosages of the formulation or version of the drug covered by that particular drug label. Note that a single drug label is commonly tied to a manufacturer's formulation: it will usually not include listings of the versions of the drug that are produced by different manufacturers, even in the same country — for that consolidated information, please see the FDA’s Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. (For more information see this link and “How to Find the Other Available Forms and Formulations of a Drug” in Step 11.)
4. Contraindications
- Reactions and pre-existing conditions that present particularly high risks for people taking the drug, including concomitant use with other psychiatric and non-psychiatric drugs.
5. Warnings & Precautions
- A selection of some of the more commonly seen or dangerous adverse effects from the drug that are considered to be serious enough to necessitate the provision of explanations and warnings.
- May include information about metabolizing the drug and “clinically important drug interactions”.
- May sometimes include information about “discontinuation syndrome” or withdrawal effects.
6. Adverse Reactions
- A selection of some of the more common adverse effects from the drug, particularly during the clinical trials that were used to get the drug approved by the FDA.
- May sometimes include information about “discontinuation syndrome” or withdrawal effects.
7. Drug Interactions
- A selection of some of the other drugs or classes/types of drugs with which the labeled drug is known to have particularly concerning or dangerous chemical interactions, such as interactions that increase or decrease the body's exposure to the labeled drug. (Sometimes requires pharmacological knowledge or use of an online drug interaction checker to determine the actual names of the specific drugs of concern.)
- May include information about drug metabolism and interactions between drugs acting on metabolic function.
8. Use in Specific Populations
- A selection of some of the information that is known about particularly notable harms that can be caused by the drug to specific, higher-risk subgroups such as pregnant women, nursing mothers, children, the elderly, and genetically predisposed poor metabolizers of certain drugs.
9. Drug Abuse and Dependence
- Information about whether the drug is considered to be prone to being used at higher doses and frequencies, or to be illicitly obtained or distributed, other than prescribed.
- May sometimes include information about the drug’s ability to cause dependence even at normally prescribed doses and frequencies.
- May sometimes include information about common symptoms of either physical dependence or addiction or both.
- May sometimes include information about common drug withdrawal symptoms.
10. Overdosage
- Information about dosage levels at which the drug may cause severely toxic or potentially fatal reactions.
11. Description
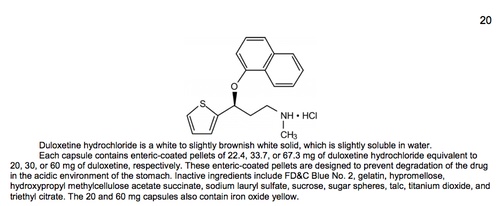
Part of the 'Description' section for Cymbalta (Last accessed May 16, 2017)
- Identifies the chemical structure of the drug and sometimes provides its full chemical/scientific name. Describes the amounts of active drug ingredient in various forms and formulations of the drug, and lists the added "inactive" ingredients such as fillers and colors.
- May indicate the relative solubility of the drug.
- May provide details about the drug’s particular modified-release formulation technologies and how these formulations are taken into the body.
12. Clinical Pharmacology
- Describes what is known or not known about how the drug “works” biologically to “treat” the conditions for which it is approved.
- Pharmacodynamics section provides a selective summary of the known biochemical information about what the drug actually does to the body.
- Pharmacokinetics section provides information about what the body does to the drug, that is, the elimination “half-life” of a drug, or how long it takes for the drug to reach peak concentrations and to be processed out of the body.
- May include more information about bioequivalence between different forms and formulations of the drug.
- Metabolism and excretion section may include some details about what is known about exactly how the drug is metabolized and eliminated by the body, including gene and enzyme-related information.
13. Nonclinical Toxicology
- Usually based on laboratory animal studies, may include a summary of what is known about the risk that that drug can cause cancer, birth defects, gene mutations, impairment of fertility, etc.
14. Clinical Studies

Part of the 'Clinical Studies' section for Cymbalta (Last accessed May 16, 2017)
- May include summaries or substantive details of the findings about the drug’s purported effectiveness, based on the key clinical trials that the manufacturer submitted to the FDA and that led to the approval of the drug for one or more specific indications. (Note: The manufacturer may have conducted studies for other indications — studies which may have revealed important information about the drug — but these studies are usually not mentioned in the drug label for the specific indication for which the drug is approved.
15. References
- Citations for information – usually not included.
16. How Supplied/Storage and Handling
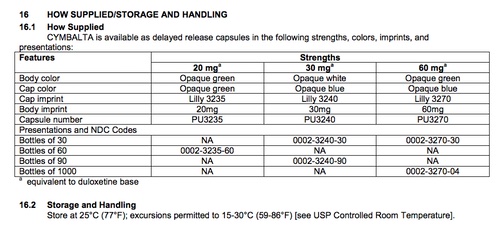
The 'How Supplied/Storage and Handling' section for Cymbalta (Last accessed May 16, 2017)
- A listing of the forms and formulations in which the drug is available. Note that a single drug label will usually not include listings of the versions of the drug that are produced by different manufacturers—for that consolidated information, please see the FDA’s Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. (For more information see this link and “How to Find the Other Available Forms and Formulations of a Drug" in Step 11.)
- Includes information about proper storage procedures, including appropriate containers and temperatures.
17. Patient Counseling Information
- Instructions to physicians on particular issues of concern about the drug that they should emphasize to patients.
+ Medication Guide and Other Information
- Newer drug labels may have a number of supplemental sections. Drug labels for some drugs are now required to include a distinct, FDA-approved “Medication Guide”, designed to summarize and help consumers understand the significance of some of the more important information about that drug. Drug labels may also include pictures of the product labels and packaging, product descriptions, lists of inactive ingredients and other information.
In this section
- Step 10- Get Informed About Your Psychiatric Drug
- Step 11- Ensuring that a Drug is Relatively ‘Taper-friendly’
- Step 12- Interactions, Reactions and Sensitivities
- Step 13- Taper Rates
- Step 14- Taper Schedules
- Step 15- Taper Methods
- Step 16- Preparatory Decisions
- Step 17- Gather the Gear
- Step 18- Essential Skills
- Step 19- Setting Up a Taper Journal
- Step 20- Implementing a Taper
TWP’s Companion Guide to Psychiatric Drug Withdrawal Part 2: Taper