Determine How ‘Taper-friendly’ Your Drug is
Make sure the information and documents you gathered in Step 10– Get Informed about Your Drug are handy, and make additional notes about your drug(s) for your records as you proceed through this step.
If you are taking a tablet or capsule, start at number 1 below.
If you are taking a manufacturer’s oral liquid, start at number 2 below.
If you are taking a drug via injection, start at number 3 below.
1 (a). Is it possible that you are taking a modified-release tablet or capsule?
“Immediate-release” (IR) or “regular-release” drugs are designed, essentially, to begin taking action “immediately” upon entering the body. Conversely, there are many different formulations of “modified-release” tablets and capsules (including for example “delayed-release” and “extended-release”) that are specially designed in ways that strictly control when and how their active ingredients are released into the body. If, prior to ingestion, a modified-release tablet is broken or the beads (often called “pellets” in drug labels) or “mini-tablets” in a modified-release capsule are altered, that release technology is damaged. This can lead to physical harm in part because the amount of active drug getting released into the bloodstream becomes unpredictable and potentially dangerous. But if you’re taking one of these formulations and want to taper, don’t despair! You have options. Continue reading, and you’ll learn how to determine whether you’re taking a modified-release drug and, if so, what your options are.
Refer to the full name of your drug as you determined it to be in Step 10, or bring out your drug bottle and/or the copy of the drug label again. If you are taking a delayed-release, extended-release, or any other kind of modified-release drug, you should see one of the following phrases or two-letter acronyms as part of the drug name:
- DR (delayed-release)
- CD (controlled-delivery)
- CR (controlled-release)
- DA (delayed-action)
- ER (extended-release)
- LA (long-acting)
- MR (modified-release)
- PA (prolonged-action)
- SA (sustained-action)
- SR (sustained-release)
- XR (extended-release)
- XL (extended-release)
If your drug name includes one of these phrases or acronyms, then you are definitely taking some kind of modified-release drug – continue reading either 1(b) or 1(c) below.
If you determine that you’re definitely not taking any kind of modified-release drug, move on to number 4 below and then the ‘Reflections’ section. If you have any doubt at all for any reason, though, then contact your pharmacist and ask if your drug is an immediate-release formulation or not. It’s a quick, simple question that will go a long way to ensuring your safety.
1 (b). If you’re taking a modified-release CAPSULE, click here.
If you’re taking a modified-release capsule, twist one open to see whether it contains beads, pellets, "chips" or mini-tablets. Laypeople in the withdrawal community report counting anywhere from 40 to over 300 tiny beads, pellets, or "chips" in a beaded capsule, and anywhere between 3-12 “mini-tablets”.

If you are taking a modified-release capsule that contains mini-tablets, in light of the small number inside each capsule along with their modified-release technology, it isn’t possible to safely taper off this particular drug formulation. People who wish to taper off this formulation can try to find a manufacturer that makes this type of capsule with small beads/pellets instead of mini-tablets. Switching to a brand-name drug requires a prescriber changing the prescription, while switching to a different generic manufacturer requires a pharmacist changing the order. People in the withdrawal community report that trying to figure out which manufacturers make capsules without mini-tablets can be difficult. It may require asking a pharmacist to contact manufacturers to specifically ask if they produce a beaded version. Alternatively, laypeople in this situation have searched to find out if there are other forms and formulations of their drug that are more ‘taper-friendly’ – For an explanation of how to do that, go to number 4 below.
If you are taking a modified-release capsule that contains beads/pellets/"chips", then tapering by either the counting-beads or weighing-beads methods are the only taper-method options possible to use at taper rates that the lay withdrawal community would consider to be careful and responsible, though.
If people taking a modified-release, beaded capsule wish to taper using any other method, then they can search for other forms and formulations of their drug that are more ‘taper-friendly’ – For an explanation of how to do that, go to number 4 below. Otherwise, move on to the ‘Reflections’ section of this Step.
1 (c) If you’re taking any kind of modified-release TABLET, click here.
For the reasons explained above, it is not safe to alter the form of any kind of modified release tablets. Therefore, in the layperson withdrawal community, it’s generally considered to be too risky to try to taper from modified-release tablets.
For its part, the FDA has indicated that in “rare instances” the FDA has approved certain modified-release tablets to be split into smaller doses along a scored line on the tablet, and has included this information in the drug labels for those specific modified-release drugs. However, tablet splitting does not allow precision control for tapering purposes and, more generally, the distribution of active drug in tablets can be inconsistent – making efforts to taper with any drug in this way especially risky and unreliable. (For more on this, see the FDA’s “Tablet Splitting: A Risky Practice” article below. As of 2018, this article is no longer available on the internet, so you have to zoom in closely to this screenshot to see the text.)
If people taking a modified-release tablet want to taper, they can search for other forms and formulations of their drug that are more “taper-friendly” – For an explanation of how to do that, go to number 4 below.
2. Are you taking a manufacturer’s oral liquid?
Tapering off a manufacturer’s oral liquid allows for more precision and control over dose levels relatively easily. While many people in the layperson withdrawal community find it easy to make their own liquid mixtures at home, some prefer to utilize the manufacturer’s existing liquid form and feel more comfortable knowing their liquid has been professionally manufactured. Step 15 of this Companion Guide describes pros and cons of this method and others.
People taking oral liquids who nevertheless want to use a different taper method that does not involve liquid can search for other forms and formulations of their drug that are also relatively 'taper-friendly' – For an explanation of how to do that, go to number 4 below.
3. Are you on an injectable psychiatric drug?
If you are taking an injectable psychiatric drug, this presents problems for tapering. Typically, people have no way to gradually taper off an injectable form of psychiatric drug on their own at home because they are likely not the one who is in control of the dosage levels. In addition, making adjustments to injectable drugs requires medical skills and high medical standards of sterility and safety.
Are you being medicated against your will with an injectable psychiatric drug?
People who are currently under a court order to take a psychiatric drug have no way to conduct a responsible taper on their own. The best that people who are being involuntarily medicated can usually hope for is to develop a better working relationship with their prescriber and get their legal status changed. They can then search for other forms and formulations of their drug that are more ‘taper-friendly’ and request to be switched to one of them. For an explanation of how to find other forms and formulations of a drug, go to number 4 below.
Are you voluntarily taking an injectable psychiatric drug?
People who are voluntarily taking an injectable form of psychiatric drug and want to taper have two possible options. They can talk with a prescriber about gradually reducing the amount of active drug in each injection in accordance with a careful, slow taper rate. Or, they can talk with a prescriber about switching to a different form of their drug from which it will be possible to taper. For an explanation of how to find other forms and formulations of a drug that are more ‘taper-friendly’, go to number 4 below.
4. How to find the other available forms and formulations of a psychiatric drug.
If you are taking a drug form or formulation that is not particularly ‘taper-friendly’ and yet still want to taper, or if for any other reason you need or want to switch from your current drug, then you will have to find out what other forms and formulations of your drug are available. Any pharmacist should be able to provide this information easily upon request. In the United States, another way to find this information is to use the free, online version of the FDA’s “Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations”.
This is the current link to the FDA’s Orange Book (if the link is broken, please contact us).
Generally, drugs that are intended to be taken orally and that are immediate- or regular-release (i.e. there is no indication in the “Dosage Form” column of the Orange Book that they are some type of modified-release) tend to be the most 'taper-friendly'. Here are some other tips on how to use the FDA’s online therapeutic equivalence tool:
- To find information about only the other forms and formulations of a brand-name version of a drug, search on the brand name (e.g. “Abilify”). To find information about all of the available generic and brand-name versions of a drug, search on the generic name or active ingredient of the drug (e.g. instead of “Abilify”, search on “Aripiprazole”).
- When viewing the search results, make sure that the box “DISCN” is unchecked – this will remove all of the discontinued versions of the drug from the results.
- In “Display”, choose the number of records to display – it’s generally best to choose “All”.
- Click on the column “Dosage Form” to order the results by the different forms of the drug that are available, such as tablets, capsules, solutions, etc. Note that the descriptions in this column also indicate clearly when the form is a type of modified-release, such as delayed-release, extended-release, etc.
- The column “Route” indicates how the particular form and formulation of the drug is intended to be taken, such as oral, intramuscular, transdermal, etc.
- Click on the column “Strength” to order the results by the different levels of dosage strength in which the drug is available.
If you’re considering switching to a different form or formulation of your drug, please proceed to number 5 below.
If you cannot find any immediate-release version of your drug, go to number 6 below.
5. Important layperson tips about switching from a modified-release to immediate-release formulation of a drug.
If you’re thinking of switching from your current drug to a different form or formulation that is more ‘taper-friendly’, then it is important to make such a transition with care. Below are some tips about this process that have been gathered from the layperson withdrawal community:
- Even without changing dosage levels, switching from one manufacturing source, form or formulation of a drug to another may produce significant and potentially problematic shifts in a person’s emotional, mental, and physical state that require becoming accustomed to. There is more information in Step 12 about these commonly occurring variations in the amounts of active drug that can be present in different versions of the same medication. Generally, one should only make a drug switch of this kind if and when fully ready, and one should allow for a period of adjustment and stabilization before beginning tapering.
- Read the drug label for the drug to learn about any important differences in how the body absorbs the modified-release and immediate-release versions, and be mindful of the effects this might cause. For example, an immediate-release version of a drug may reach peak concentrations in the bloodstream hours sooner than an extended release version. See "TWP’s Guide to the FDA-approved Drug Label" for tips to finding this information.
- Consult with both a well-informed pharmacist and a prescriber to determine what the optimal dosing schedule would be once the switch from modified- to immediate-release is made. For example, many people who have been on modified-release formulations taken once a day find that when they switch to the immediate-release formulation, they need to dose twice a day in order to keep the blood levels of their drug consistent with what they previously were.
6. What if I can’t find any 'taper-friendly' version of my drug?
It is sometimes the case that a particular drug meets two criteria that together, in the opinion of most people in the lay withdrawal community, make reasonably safe tapering with the drug virtually impossible: the drug comes in the form of a modified-release tablet AND there is no immediate-release alternative to it. (Two U.S. drugs that as of May 2017 meet these criteria, for example, are desvenlafaxine (Pristiq) and paliperidone (Invega). People in this situation who still want to taper have two options:
- They can ask a pharmacist if it’s possible to compound their drug into smaller dose sizes that could be tapered from. See Step 15 for more information about compounding.
- They can discuss the situation with a well-informed pharmacist and prescriber, and ask for help to “bridge” off their current drug and onto an immediate-release formulation of a different drug that is as chemically similar as possible. For example, desvenlafaxine (Pristiq) is an active metabolite of venlafaxine (Effexor), and paliperidone (Invega) is an active metabolite of risperidone (Risperdal), and some people have reported success bridging from desvenlafaxine to venlafaxine or from paliperidone to risperidone and then beginning to taper. It is generally essential to research and discuss such possible changes in collaboration with a well-informed pharmacist, and a prescriber will also have to be involved if a new prescription is required. People in online withdrawal forums can also be invaluable assets for learning about exactly how others have successfully bridged.
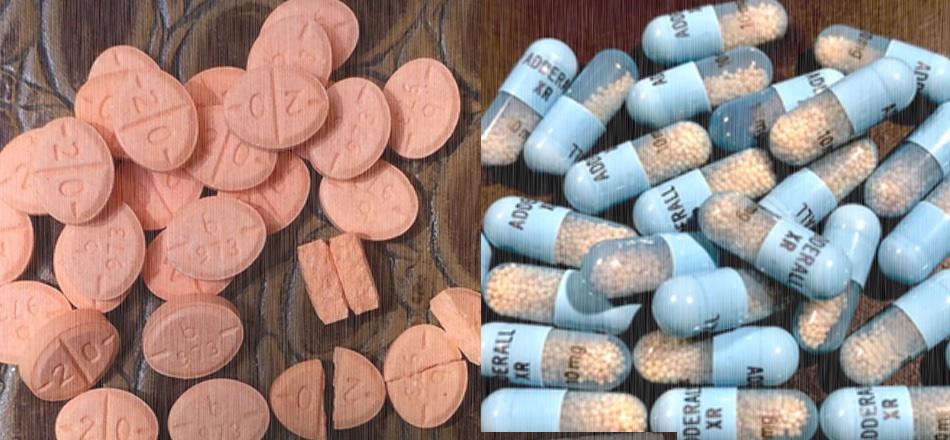
Image of Adderall tablets courtesy of Seppi333 and Creative Commons. Image of Adderall XR capsules courtesy of Benjamin Vincent Kasapoglu and Creative Commons. These images have been combined, and modified stylistically.
In this section
- Step 10- Get Informed About Your Psychiatric Drug
- Step 11- Ensuring that a Drug is Relatively ‘Taper-friendly’
- Step 12- Interactions, Reactions and Sensitivities
- Step 13- Taper Rates
- Step 14- Taper Schedules
- Step 15- Taper Methods
- Step 16- Preparatory Decisions
- Step 17- Gather the Gear
- Step 18- Essential Skills
- Step 19- Setting Up a Taper Journal
- Step 20- Implementing a Taper
TWP’s Companion Guide to Psychiatric Drug Withdrawal Part 2: Taper